| medXchange home |
Patient-Centric Electronic Medical Record |
Patient Centric Meta Database |
Healthcare Sectors |
| Applications & Technology |
Data Protection | News & Media | Company Information |
European Data Protection Regulations |
Compliance to Regualtions & StandardsmedXchange complies with the strictest data protection laws and regulations covering the protection of personal health and medical data. Positive opinions will be received by all European national data protection authorities and in particular the Swiss Federal Data Protection Commissioner and CNIL, the French Data Protection Commission. |
| US National Research Council |
In March of 1997, the US National Research Council, NRC of the US National Academy
of Sciences issued the report, "For the Record: Protecting Electronic Health
Information". Thirteen technical implementation practices were recommended.
|
| US HIPAA |
The medXchange Meta Database and services
comply with the US Health Insurance Portability and Accountability Act of 1996,
HIPAA Administrative Simplification. |
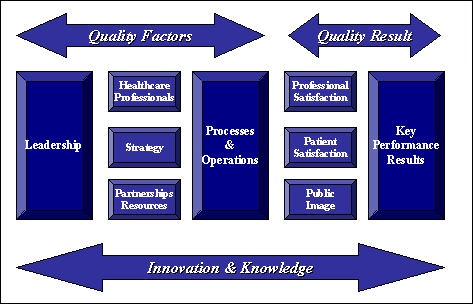
| Quality Standards |
The "Projet Dossier Patient 2003" initiated by the Swiss University Hospitals has
issued a quality standard evaluation document "Standards de qualité pour le dossier
patient informatisé" (Quality standards for electronic patient records). Nine areas
are evaluated which deal with business strategy, management commitment, partnerships,
technology strategy, operations, patient and healthcare professional satisfaction,
and effect on healthcare.
|
|
This Page was created on 08.11.2001 and last modified on 11.04.2025
Our Website does not host any form of advertisement
|